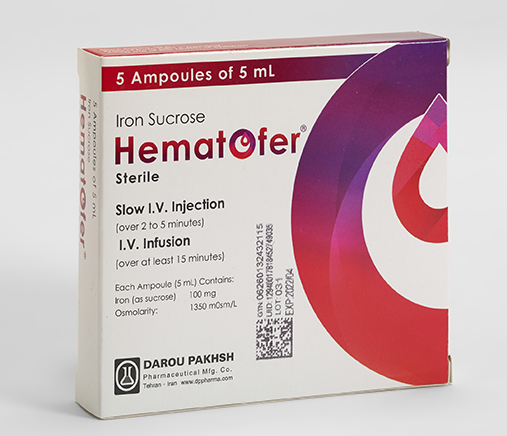
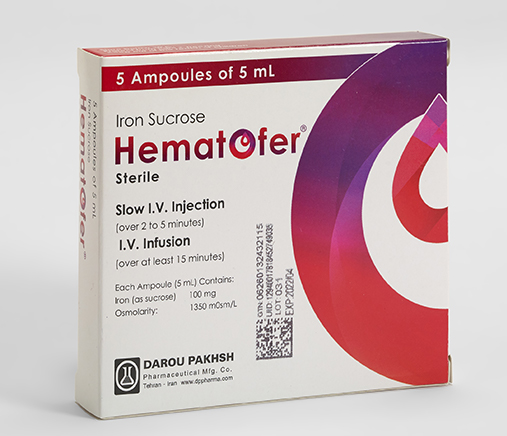
Iron Sucrose
- Iron Sucrose
- Hematofer®
- Solution and Powder for Injection
- 20mg/mL
- Ampoule
- 5mL
Each Ampoule (5mL) Contains:
Iron Sucrose 100mg
Osmolarity:
1350mOsm/L
Iron Sucrose 100mg
Osmolarity:
1350mOsm/L
• Iron Deficiency Anemia
Mechanism of Action
Replacement of iron stores found in hemoglobin, myoglobin, and enzymes, works to transport oxygen via hemoglobin
Pharmacokinetics
Excretion: Urine (5%)
Contraindications
• Hypersensitivity
• Anemia not caused by iron deficiency
• Iron overload
Adverse Reactions
Hypotension, Muscle cramps, Headache, Nausea, Dizziness, Fatigue, Arthralgia, Back pain, Hypertension, Fluid overload, Peripheral edema, Cough, Vomiting, Diarrhea, Constipation, Pruritus Test
Major Drug Interactions
-
Warnings
• Withhold therapy in tissue iron overload
Recommendations for Patient
• This medication is given by injection into a vein as directed by your doctor. It is usually given slowly over 2 to 5 minutes or it can also be mixed in a saline solution and given over a longer time.
• Iron sucrose may rarely cause a serious allergic reaction. Careful monitoring during and at least 30 minutes after your treatment may decrease your risk.
Pregnancy Considerations
Category: B
Breastfeeding Considerations
No special precautions are required.
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: Maybe acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk.
C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done.
D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk.
X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist.
NA: Information not available.

Replacement of iron stores found in hemoglobin, myoglobin, and enzymes, works to transport oxygen via hemoglobin
Pharmacokinetics
Excretion: Urine (5%)
Contraindications
• Hypersensitivity
• Anemia not caused by iron deficiency
• Iron overload
Adverse Reactions
Hypotension, Muscle cramps, Headache, Nausea, Dizziness, Fatigue, Arthralgia, Back pain, Hypertension, Fluid overload, Peripheral edema, Cough, Vomiting, Diarrhea, Constipation, Pruritus Test
Major Drug Interactions
-
Warnings
• Withhold therapy in tissue iron overload
Recommendations for Patient
• This medication is given by injection into a vein as directed by your doctor. It is usually given slowly over 2 to 5 minutes or it can also be mixed in a saline solution and given over a longer time.
• Iron sucrose may rarely cause a serious allergic reaction. Careful monitoring during and at least 30 minutes after your treatment may decrease your risk.
Pregnancy Considerations
Category: B
Breastfeeding Considerations
No special precautions are required.
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: Maybe acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk.
C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done.
D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk.
X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist.
NA: Information not available.
Send to other people